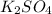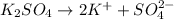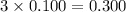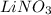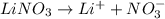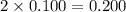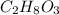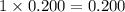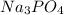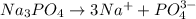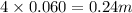Chemistry, 20.12.2019 20:31 bronkosarecool
Choose the aqueous solution that has the highest boiling point. these are all solutions of nonvolatile solutes and you should assume ideal van't hoff factors where applicable. a. 0.100 m k2so4 b. 0.100 m lino3 c. 0.200 m c3h8o3 d. 0.060 m na3po4 e. they all have the same boiling point.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
Choose the aqueous solution that has the highest boiling point. these are all solutions of nonvolati...
Questions


Mathematics, 16.12.2021 06:00

Mathematics, 16.12.2021 06:00

Mathematics, 16.12.2021 06:00

Mathematics, 16.12.2021 06:00


History, 16.12.2021 06:00

Mathematics, 16.12.2021 06:00

Physics, 16.12.2021 06:00



Mathematics, 16.12.2021 06:00


Mathematics, 16.12.2021 06:00


Biology, 16.12.2021 06:00

Mathematics, 16.12.2021 06:00


Mathematics, 16.12.2021 06:00

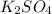
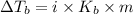
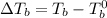 = Elevation in boiling point
= Elevation in boiling point 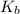 = boiling point constant
= boiling point constant