Chemistry, 20.12.2019 20:31 wrightstephanie193
Agalvanic (voltaic) cell consists of an electrode composed of titanium in a 1.0 m titanium(ii) ion solution and a second electrode composed of tin in a 1.0 m tin(ii) ion solution, connected by a salt bridge. calculate the standard potential for this cell at 25c.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2


Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

You know the right answer?
Agalvanic (voltaic) cell consists of an electrode composed of titanium in a 1.0 m titanium(ii) ion s...
Questions

History, 27.09.2019 18:30


History, 27.09.2019 18:30

Biology, 27.09.2019 18:30


Mathematics, 27.09.2019 18:30

History, 27.09.2019 18:30


Physics, 27.09.2019 18:30


History, 27.09.2019 18:30

Health, 27.09.2019 18:30

Biology, 27.09.2019 18:30

Mathematics, 27.09.2019 18:30

Mathematics, 27.09.2019 18:30



Mathematics, 27.09.2019 18:30


![E^0_{[Sn^{2+}/Sn]}=-0.14V](/tpl/images/0428/1023/81a51.png)
![E^0_{[Ti^{2+}/Ti]}=-1.63V](/tpl/images/0428/1023/456c4.png)
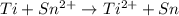
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Ti^{2+}]}{[Sn^{2+}]}](/tpl/images/0428/1023/a72a7.png)
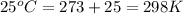
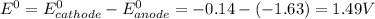
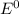 are standard reduction potentials.
are standard reduction potentials.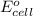 = standard electrode potential of the cell = 1.49 V
= standard electrode potential of the cell = 1.49 V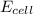 = emf of the cell = ?
= emf of the cell = ?