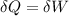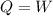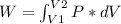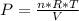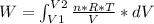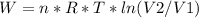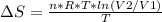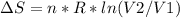Chemistry, 20.12.2019 19:31 Mattixwillard
An ideal gas contained in a piston-cylinder assembly is compressed isothermally in an internally reversible process.
(a) determine if the entropy change of the gas is greater than, equal to or less than zero, justify your answer
(b) determine if for the same change of state, the entropy change for an irreversible process is greater than, equal to or less than part (a)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
An ideal gas contained in a piston-cylinder assembly is compressed isothermally in an internally rev...
Questions

Social Studies, 12.02.2022 08:20


Mathematics, 12.02.2022 08:20



Social Studies, 12.02.2022 08:20

Mathematics, 12.02.2022 08:20


Mathematics, 12.02.2022 08:20

Biology, 12.02.2022 08:20

Business, 12.02.2022 08:20


Biology, 12.02.2022 08:20

Chemistry, 12.02.2022 08:20

Biology, 12.02.2022 08:20

English, 12.02.2022 08:20



Mathematics, 12.02.2022 08:20

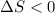
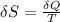
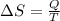
![\delta U=[tex]\delta Q- \delta W](/tpl/images/0427/9845/795a2.png)
![0=[tex]\delta Q- \delta W](/tpl/images/0427/9845/24655.png)