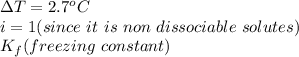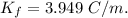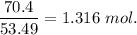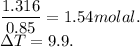Chemistry, 20.12.2019 19:31 savyblue1724707
When 70.4 g of benzamide (c7h7no) are dissolved in 850. g of a certain mystery liquid x , the freezing point of the solution is 2.7°c lower than the freezing point of pure x. on the other hand, when 70.4 g of ammonium chloride (nh4ci) are dissolved in the same mass of x, the freezing point of the solution is 9.9 °c lower than the freezing point of pure x.
a) calculate the van't hoff factor for ammonium chloride in x. be sure your answer has a unit symbol, if necessary, and round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
When 70.4 g of benzamide (c7h7no) are dissolved in 850. g of a certain mystery liquid x , the freezi...
Questions



Mathematics, 06.07.2021 18:30

Mathematics, 06.07.2021 18:30


Mathematics, 06.07.2021 18:30

Mathematics, 06.07.2021 18:30

English, 06.07.2021 18:30

Mathematics, 06.07.2021 18:30

Mathematics, 06.07.2021 18:30

History, 06.07.2021 18:30

Mathematics, 06.07.2021 18:30






Mathematics, 06.07.2021 18:30

Mathematics, 06.07.2021 18:30

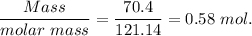
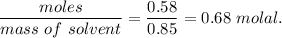
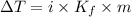 .....1
.....1