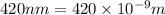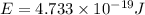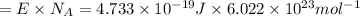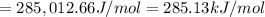Chemistry, 20.12.2019 18:31 pablogonzaleztellez
An important reaction in the formation of photochemical smog is the photodissociation of no2: no2 + hv > > > no(g) + o(g) the maximum wavelength of light that can cause this reaction is 420 nm. a) in what part of the electromagnetic spectrum is light with this wavelength found? b) what is the maximum strength of a bond, in kj/mol, that can be broken by absorption of a photon of 420-nm light? c) write out the photodissociation reaction showing lewis-dot structures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
You know the right answer?
An important reaction in the formation of photochemical smog is the photodissociation of no2: no2 +...
Questions

Spanish, 08.07.2019 22:30


Chemistry, 08.07.2019 22:30

Social Studies, 08.07.2019 22:30

Mathematics, 08.07.2019 22:30

Mathematics, 08.07.2019 22:30

History, 08.07.2019 22:30

Mathematics, 08.07.2019 22:30

History, 08.07.2019 22:30


Physics, 08.07.2019 22:30

Mathematics, 08.07.2019 22:30


Biology, 08.07.2019 22:30






Biology, 08.07.2019 22:30

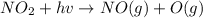
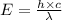
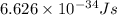
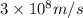
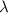 = wavelength =
= wavelength =