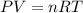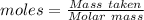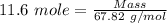Chemistry, 20.12.2019 18:31 hannabeth91
Boron trifluoride gas is collected at 2.0 degree c in an evacuated flask with a measured volume of 15.0 l. when all the gas has been collected, the pressure in the flask is measured to be 0.130 atm. calculate the mass and number of moles of boron trifluoride gas that were collected. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 23.06.2019 09:20
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2

Chemistry, 23.06.2019 11:30
All of the following describe uses of nonrenewable energy sources except
Answers: 3
You know the right answer?
Boron trifluoride gas is collected at 2.0 degree c in an evacuated flask with a measured volume of 1...
Questions


Biology, 29.07.2019 13:00



History, 29.07.2019 13:10





Law, 29.07.2019 13:10


Health, 29.07.2019 13:10

Computers and Technology, 29.07.2019 13:10

Social Studies, 29.07.2019 13:10

History, 29.07.2019 13:10

Social Studies, 29.07.2019 13:10

Mathematics, 29.07.2019 13:10

History, 29.07.2019 13:10

English, 29.07.2019 13:10