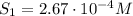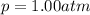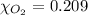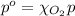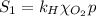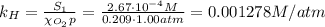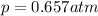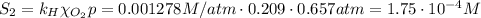The solubility of oxygen in lakes high in the rocky mountains is affected by the altitude. if the solubility of o2 from the air is 2.67 ✕ 10-4 m at sea level and 25°c, what is the solubility of o2 at an elevation of 12,000 ft where the atmospheric pressure is 0.657 atm? assume the temperature is 25°c, and that the mole fraction of o2 in air is 0.209 at both 12,000 ft and at sea level.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
The solubility of oxygen in lakes high in the rocky mountains is affected by the altitude. if the so...
Questions







History, 12.12.2021 23:10

Mathematics, 12.12.2021 23:10



History, 12.12.2021 23:10

Mathematics, 12.12.2021 23:10





Biology, 12.12.2021 23:20

Mathematics, 12.12.2021 23:20


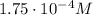
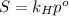
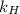 is Henry's law constant.
is Henry's law constant.