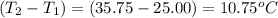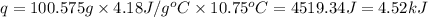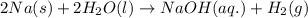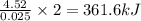Chemistry, 20.12.2019 18:31 King1Gates
Sodium metal reacts with water to produce hydrogen gas and sodium hydroxide according to the chemical equation shown below.
when 0.0 25 mol of na is added to 100.00 g of water, the temperature of the resulting solution rises from 25.00°c to 35.75°c.
if the specific heat of the solution is 4.18 j/(g · °c), calculate δh for the reaction, as written.
2 na(s) + 2 h2o(l) → 2 naoh(aq) + h2(g) δh= ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2


Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
Sodium metal reacts with water to produce hydrogen gas and sodium hydroxide according to the chemica...
Questions

Computers and Technology, 23.07.2019 21:00


Computers and Technology, 23.07.2019 21:00

Chemistry, 23.07.2019 21:00

Biology, 23.07.2019 21:00



Social Studies, 23.07.2019 21:00

Mathematics, 23.07.2019 21:00

Geography, 23.07.2019 21:00

Health, 23.07.2019 21:00

Health, 23.07.2019 21:00

Health, 23.07.2019 21:00





English, 23.07.2019 21:00

Mathematics, 23.07.2019 21:00

English, 23.07.2019 21:00

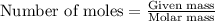
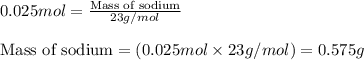
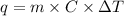
 = change in temperature =
= change in temperature =