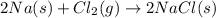Which of the following statements is not true?
a) when two nonmetals react, the compound formed is ionic.
b) two nonmetals can undergo an oxidation-reduction reaction.
c) a metal-nonmetal reaction can always be assumed to be an oxidation-reduction reaction.
d) a metal-nonmetal reaction involves electron transfer.
e) when a metal reacts with a nonmetal, an ionic compound is formed.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1


Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1

You know the right answer?
Which of the following statements is not true?
a) when two nonmetals react, the compound for...
a) when two nonmetals react, the compound for...
Questions



Mathematics, 16.02.2021 05:30





Mathematics, 16.02.2021 05:30




Mathematics, 16.02.2021 05:30

Chemistry, 16.02.2021 05:30

History, 16.02.2021 05:30



Mathematics, 16.02.2021 05:30


Mathematics, 16.02.2021 05:30