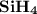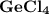1.the element silicon would be expected to covalent bond(s) in order to obey the octet rule.
use the octet rule to predict the formula of the compound that would form between silicon and hydrogen , if the molecule contains only one silicon atom and only single bonds are formed.
formula:
2. the element germanium would be expected to covalent bond(s) in order to obey the octet rule. use the octet rule to predict the formula of the compound that would form between germanium and chlorine , if the molecule contains only one germanium atom and only single bonds are formed.
formula:

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 23.06.2019 13:30
Asap at one point in history people could measure temperature by looking at the volume of a sample of gas. suppose a sample in a gas thermometer has a volume of 135ml at 11.0°c. indicate what temperature would correspond to each of the following volumes: 113 ml, and 155 ml.
Answers: 1

Chemistry, 23.06.2019 18:30
Describe two techniques used to measure the ph of a solution
Answers: 2
You know the right answer?
1.the element silicon would be expected to covalent bond(s) in order to obey the octet rule.
<...
<...
Questions

Social Studies, 01.08.2019 13:47

Physics, 01.08.2019 13:47

Spanish, 01.08.2019 13:47



Mathematics, 01.08.2019 13:47


Social Studies, 01.08.2019 13:47


English, 01.08.2019 13:47


Social Studies, 01.08.2019 13:47



Social Studies, 01.08.2019 13:47

Mathematics, 01.08.2019 13:47

Mathematics, 01.08.2019 13:47

History, 01.08.2019 13:47