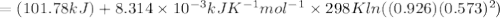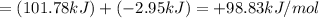Chemistry, 20.12.2019 05:31 birdman37361
Achemist fills a reaction vessel withmercurous chloridesolid, mercury (i)aqueous solution, andchlorideaqueous solution at a temperature of. under these conditions, calculate the reaction free energyfor the following chemical reaction: use the thermodynamic information in the aleks data tab. round your answer to the nearest kilojoule.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

You know the right answer?
Achemist fills a reaction vessel withmercurous chloridesolid, mercury (i)aqueous solution, andchlori...
Questions



Geography, 14.10.2019 15:00




English, 14.10.2019 15:00


Health, 14.10.2019 15:00



Social Studies, 14.10.2019 15:00

Mathematics, 14.10.2019 15:00

Mathematics, 14.10.2019 15:00


English, 14.10.2019 15:00


Biology, 14.10.2019 15:00

Mathematics, 14.10.2019 15:00

Biology, 14.10.2019 15:00

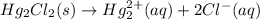
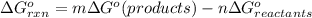
![=[(1\times \Delta G^{o}_{f(Hg_{2}^{2+})})+(2\times \Delta G^{o}_{f(Cl^{-})})-(1\times \Delta G^{o}_{f(Hg_{2}Cl_{2})})]](/tpl/images/0427/2894/94afc.png)
![=[(1\times 153.5+2\times -131.25)-(1\times -210.78)]kJ=+101.78kJ](/tpl/images/0427/2894/80b68.png)
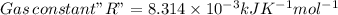
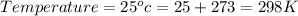
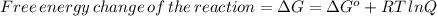
![\Delta G=\Delta G^{o}+RT\,ln([Hg_{2}^{2+}][Cl^{-}]^{2})](/tpl/images/0427/2894/6eb91.png)