
Asystem undergoes a two-step process. in step 1, it absorbs 50. j of heat at constant volume. in step 2, it releases 5j of heat at 1 atm. as it returned to its original internal energy. find the change in the volume of the system during the second step and identify it as an expansion or compression.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2


Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
Asystem undergoes a two-step process. in step 1, it absorbs 50. j of heat at constant volume. in ste...
Questions

History, 06.10.2019 20:00

Mathematics, 06.10.2019 20:00

History, 06.10.2019 20:00

History, 06.10.2019 20:00


Mathematics, 06.10.2019 20:00



Mathematics, 06.10.2019 20:00

Mathematics, 06.10.2019 20:00

Social Studies, 06.10.2019 20:00

Biology, 06.10.2019 20:00



Biology, 06.10.2019 20:00




History, 06.10.2019 20:00

Business, 06.10.2019 20:00

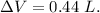
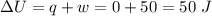
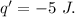
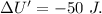
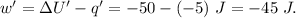
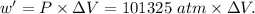
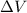 is positive.
is positive.


