Chemistry, 20.12.2019 03:31 brutalgitaffe
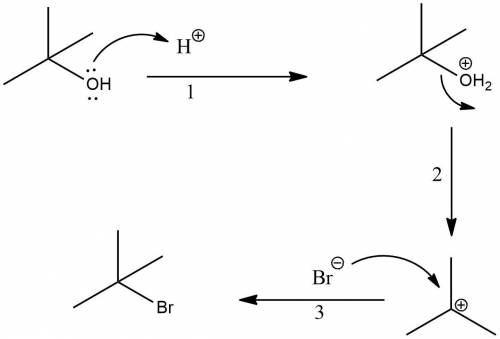
Butyl bromide (2-brom-2-methylpropane) can be prepared by simply taking t-butyl alcohol and shaking it with an aqueous solution of hbr at room temperature. the reaction is much faster than with n-butyl alcohol and is essentially 100% complete within a few minutes. give a mechanism for this reaction. note: it is not the same mechanism as for the lab preparation of 1-bromobutane. what is this reaction called?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

You know the right answer?
Butyl bromide (2-brom-2-methylpropane) can be prepared by simply taking t-butyl alcohol and shaking...
Questions

Chemistry, 04.03.2021 21:00

Biology, 04.03.2021 21:00

Mathematics, 04.03.2021 21:00


Chemistry, 04.03.2021 21:00


Mathematics, 04.03.2021 21:00

English, 04.03.2021 21:00

Mathematics, 04.03.2021 21:00

Advanced Placement (AP), 04.03.2021 21:00

Mathematics, 04.03.2021 21:00

History, 04.03.2021 21:00






Geography, 04.03.2021 21:00

Chemistry, 04.03.2021 21:00

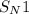 (substitution nucleophilic bimolecular) reaction
(substitution nucleophilic bimolecular) reaction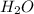 .
.