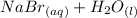Chemistry, 20.12.2019 03:31 dukkchild666
Avolume of 50.0 ml of 0.400 m hbr at 24.35°c is added to 50.0 ml of 0.400 m naoh, also at 24.35°c. after the reaction, the final temperature is 27.06°c. calculate the enthalpy change, h, per mole of hbr (in kj) for the reaction. heat specific capacity of the solution is 4.184 j/oc, density of the solution is 1.0 g/ml.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Avolume of 50.0 ml of 0.400 m hbr at 24.35°c is added to 50.0 ml of 0.400 m naoh, also at 24.35°c. a...
Questions



Mathematics, 01.12.2019 11:31

World Languages, 01.12.2019 11:31

Mathematics, 01.12.2019 11:31


English, 01.12.2019 11:31

History, 01.12.2019 11:31

Mathematics, 01.12.2019 11:31

Chemistry, 01.12.2019 11:31

English, 01.12.2019 11:31

Mathematics, 01.12.2019 11:31

Business, 01.12.2019 11:31

Biology, 01.12.2019 11:31

Biology, 01.12.2019 11:31

Computers and Technology, 01.12.2019 11:31




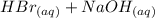 ⇒
⇒