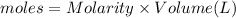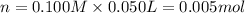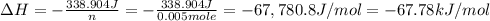Chemistry, 20.12.2019 03:31 cancerbaby209
When 50.0 ml of 0.100 m agno3 is mixed with 50.0 ml of 0.100 m hcl in a coffee cup calorimeter, the temperature increases from 23.40 °c to 24.21°c. if the specific heat of the solution is 4.184 j/°c·g and its density is 1.00 g/ml, assuming that the calorimeter undergoes no heat change, calculate the enthalpy change for the following reaction in units of kj/mol agno3: agno3(aq) + hcl(aq) à agcl(s) + hno3(aq)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
When 50.0 ml of 0.100 m agno3 is mixed with 50.0 ml of 0.100 m hcl in a coffee cup calorimeter, the...
Questions



English, 27.01.2020 23:31



Biology, 27.01.2020 23:31


Mathematics, 27.01.2020 23:31


Mathematics, 27.01.2020 23:31


Social Studies, 27.01.2020 23:31

Mathematics, 27.01.2020 23:31

Mathematics, 27.01.2020 23:31

History, 27.01.2020 23:31



Mathematics, 27.01.2020 23:31


Mathematics, 27.01.2020 23:31

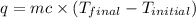
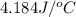
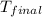 = final temperature =
= final temperature = 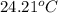
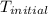 = initial temperature =
= initial temperature = 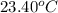
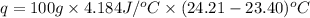
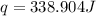
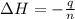
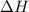 = enthalpy change = ?
= enthalpy change = ?