
Chemistry, 20.12.2019 02:31 ameera1973
1.00 liter solution contains 0.40 m acetic acid and 0.52 m potassium acetate. if 0.130 moles of calcium hydroxide are added to this system, indicate whether the following statements are true or false. (assume that the volume does not change upon the addition of calcium hydroxide.)
a. the number of moles of ch3cooh will increase.
b. the number of moles of ch3coo- will decrease.
c. the equilibrium concentration of h3o will remain the same.
d. the ph will decrease.
e. the ratio of [ch3cooh] / [ch3coo-] will increase.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1


Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
You know the right answer?
1.00 liter solution contains 0.40 m acetic acid and 0.52 m potassium acetate. if 0.130 moles of calc...
Questions



Computers and Technology, 24.04.2020 01:52


Computers and Technology, 24.04.2020 01:52



Mathematics, 24.04.2020 01:52

History, 24.04.2020 01:52

Arts, 24.04.2020 01:52

English, 24.04.2020 01:52

Mathematics, 24.04.2020 01:52

Mathematics, 24.04.2020 01:52


English, 24.04.2020 01:52

Mathematics, 24.04.2020 01:52



History, 24.04.2020 01:52

Mathematics, 24.04.2020 01:52

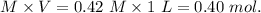
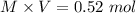
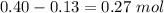
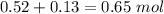
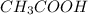 decrease and
decrease and 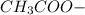 increase.
increase.

