Chemistry, 20.12.2019 01:31 maelaysiap
Carbon monoxide (co) is toxic because it binds more strongly to the iron in hemoglobin (hb) than does oxygen (o2), as indicated by these approximate standard free-energy changes in blood:
reaction a: reaction b: hb+o2hb+co⟶⟶hbo2,hbco, δg∘=−70 kj/mol δg∘=−80 kj/mol
estimate the equilibrium constant k at 298 k for the equilibrium
hbo2+co⇌hbco+o2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3
You know the right answer?
Carbon monoxide (co) is toxic because it binds more strongly to the iron in hemoglobin (hb) than doe...
Questions

Mathematics, 20.10.2020 22:01


Chemistry, 20.10.2020 22:01


English, 20.10.2020 22:01


English, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

Computers and Technology, 20.10.2020 22:01

Social Studies, 20.10.2020 22:01

Health, 20.10.2020 22:01

Chemistry, 20.10.2020 22:01




World Languages, 20.10.2020 22:01

Computers and Technology, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

English, 20.10.2020 22:01

History, 20.10.2020 22:01

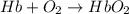 ;
; 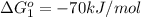
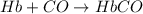 ;
; 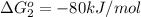
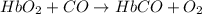 ;
; 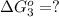
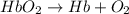 ;
; 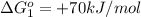
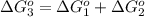
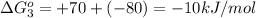
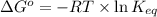
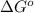 = standard Gibbs free energy = -10kJ/mol = -10000 J/mol
= standard Gibbs free energy = -10kJ/mol = -10000 J/mol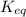 = equilibrium constant = ?
= equilibrium constant = ?