
The spontaneous reaction that occurs when the cell in the picture operates is as follows: 2ag+ + cd(s) 2 ag(s) + cd2+ (a) voltage increases. (b) voltage decreases but remains > zero. (c) voltage becomes zero and remains at zero. (d) no change in voltage occurs. (e) direction of voltage change cannot be predicted without additional information. which of the above occurs for each of the following circumstances? 14. a 50-milliliter sample of a 2-molar cd(no3)2 solution is added to the left beaker. 15. the silver electrode is made larger. 16. the salt bridge is replaced by a platinum wire. 17. current is allowed to flow for 5 minutes.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
The spontaneous reaction that occurs when the cell in the picture operates is as follows: 2ag+ + cd...
Questions


Law, 09.12.2020 01:40


English, 09.12.2020 01:40


Mathematics, 09.12.2020 01:40

Mathematics, 09.12.2020 01:40

Social Studies, 09.12.2020 01:40

Mathematics, 09.12.2020 01:40

Mathematics, 09.12.2020 01:40

History, 09.12.2020 01:40

Biology, 09.12.2020 01:40


Mathematics, 09.12.2020 01:40

Arts, 09.12.2020 01:40





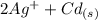 ⇒
⇒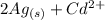
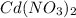 solution is added to the left beaker.
solution is added to the left beaker. 

