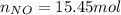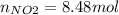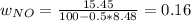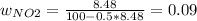Chemistry, 20.12.2019 00:31 ashleypere99
Consider the reactions _1 2 n2(g) + _1 2 o2(g) → no(g) _1 2 n2(g) + o2(g) → no2(g) if these reactions come to equilibrium after combustion in an internal-combustion engine at 2000 k and 200 bar, estimate the mole fractions of no and no2 present for mole fractions of nitrogen and oxygen in the combustion products of 0.70 and 0.05.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1
You know the right answer?
Consider the reactions _1 2 n2(g) + _1 2 o2(g) → no(g) _1 2 n2(g) + o2(g) → no2(g) if these reaction...
Questions

Mathematics, 06.11.2020 07:40

Physics, 06.11.2020 07:40

Mathematics, 06.11.2020 07:40

English, 06.11.2020 07:40

Physics, 06.11.2020 07:40




Mathematics, 06.11.2020 07:40

English, 06.11.2020 07:40

Mathematics, 06.11.2020 07:40

Mathematics, 06.11.2020 07:40


Physics, 06.11.2020 07:40

History, 06.11.2020 07:40

Mathematics, 06.11.2020 07:40

Biology, 06.11.2020 07:40


Chemistry, 06.11.2020 07:40

Mathematics, 06.11.2020 07:40

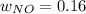
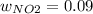
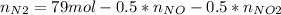
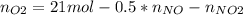
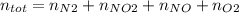
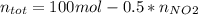
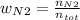
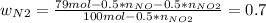
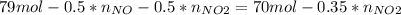
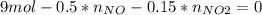 (1)
(1)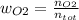
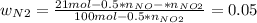
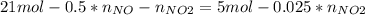
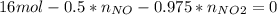 (2)
(2)