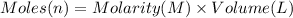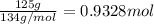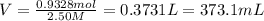You work for a cutlery manufacturer who wants to electrolytically precipitate 0.500 g of silver onto each piece of a batch of 250 forks. the preferred electrolytic solution for silver is agcn(aq). aqueous agcn is purchased as a 2.50 m solution. how many ml of agcn(aq) must be poured into your electrolysis vat to ensure you have sufficient ag to plate all of the forks

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
You work for a cutlery manufacturer who wants to electrolytically precipitate 0.500 g of silver onto...
Questions


Mathematics, 07.11.2020 21:00

English, 07.11.2020 21:00


Mathematics, 07.11.2020 21:00



English, 07.11.2020 21:00

Mathematics, 07.11.2020 21:00


Mathematics, 07.11.2020 21:00

Mathematics, 07.11.2020 21:00




World Languages, 07.11.2020 21:10

Mathematics, 07.11.2020 21:10

English, 07.11.2020 21:10

Mathematics, 07.11.2020 21:10