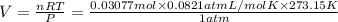Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3

You know the right answer?
Zinc will react with hydrochloric acid to produce hydrogen gas. zn(s)+2hcl(aq)⟶zncl2(aq)+h2(g)what i...
Questions




Biology, 06.04.2020 08:30

Mathematics, 06.04.2020 08:30






Mathematics, 06.04.2020 08:30

Mathematics, 06.04.2020 08:30


Mathematics, 06.04.2020 08:30


Social Studies, 06.04.2020 08:30

Computers and Technology, 06.04.2020 08:31

Biology, 06.04.2020 08:31

Advanced Placement (AP), 06.04.2020 08:31

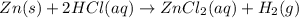
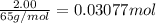
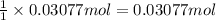 of hydrogen gas
of hydrogen gas