

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
25 points
ammonia (nh3) reacts with oxygen (o2) to produce nitrogen monoxide (no) and water (h2...
ammonia (nh3) reacts with oxygen (o2) to produce nitrogen monoxide (no) and water (h2...
Questions


History, 12.03.2020 06:58


History, 12.03.2020 06:58

History, 12.03.2020 06:58

Mathematics, 12.03.2020 06:58





Mathematics, 12.03.2020 06:59

Mathematics, 12.03.2020 06:59

English, 12.03.2020 06:59


Physics, 12.03.2020 06:59

Physics, 12.03.2020 06:59



Mathematics, 12.03.2020 06:59

History, 12.03.2020 06:59

 is
is 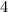 ; the coefficient in front of
; the coefficient in front of 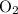 is
is 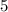 . The ratio between these two coefficients is equal to
. The ratio between these two coefficients is equal to  .
.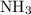 is consumed. The ratio between the number of moles of
is consumed. The ratio between the number of moles of  . That is:
. That is: .
. .
.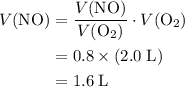 .
.

