
Chemistry, 19.12.2019 04:31 hilarydodard7099
Refrigerant-134a enters the coils of the evaporator of a refrigeration system as a saturated liquid-vapor mixture at a pressure of 160 kpa. the refrigerant absorbs 180 kj/kg of heat from the cooled space, which is maintained at -5°c, and leaves as a saturated vapor at the same pressure. determine
(a) the entropy change of the refrigerant,
(b) the entropy change of the cooled space, and
(c) the total entropy change for this process.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2
You know the right answer?
Refrigerant-134a enters the coils of the evaporator of a refrigeration system as a saturated liquid-...
Questions

Mathematics, 24.09.2020 14:01

Mathematics, 24.09.2020 14:01


Mathematics, 24.09.2020 14:01



Chemistry, 24.09.2020 14:01





Mathematics, 24.09.2020 14:01

Mathematics, 24.09.2020 14:01


Mathematics, 24.09.2020 14:01

History, 24.09.2020 14:01

Mathematics, 24.09.2020 14:01


Mathematics, 24.09.2020 14:01

Mathematics, 24.09.2020 14:01

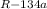 ) = Q/T
) = Q/T
 ) of the cooled space (ΔS
) of the cooled space (ΔS
 ) = ΔS
) = ΔS

