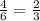Chemistry, 19.12.2019 01:31 asiababbie33
The al2o3 crystal structure (corundum) consists of an hcp arrangement of o2- ions; the al3+ ions occupy octahedral positions. what fraction of the available octahedral positions are filled with al3+ ions? in corundum, the al3+ ions do not fill the tetrahedral positions.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1


Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
The al2o3 crystal structure (corundum) consists of an hcp arrangement of o2- ions; the al3+ ions oc...
Questions

Chemistry, 21.08.2020 01:01

Mathematics, 21.08.2020 01:01


Mathematics, 21.08.2020 01:01






Chemistry, 21.08.2020 01:01







Computers and Technology, 21.08.2020 01:01



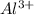 ions is
ions is 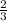
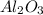 crystal structure consists of an HCP arrangement of
crystal structure consists of an HCP arrangement of 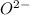 ions.
ions.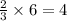
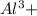 ions are present = 4
ions are present = 4