
Chemistry, 18.12.2019 20:31 12233445566
The ka of carbonic acid (h2co3) is 4.3 x 10–7. a solution of sodium hydrogen carbonate (nahco3) solution is created by dissolving 4.00 moles of sodium hydrogen carbonate in 2.00 l of aqueous solution. what is the ph of the solution at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
The ka of carbonic acid (h2co3) is 4.3 x 10–7. a solution of sodium hydrogen carbonate (nahco3) solu...
Questions

History, 30.07.2019 04:00








Spanish, 30.07.2019 04:00


History, 30.07.2019 04:00

Mathematics, 30.07.2019 04:00

History, 30.07.2019 04:00

Biology, 30.07.2019 04:00




English, 30.07.2019 04:00


History, 30.07.2019 04:00

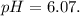
 .
. , M=
, M=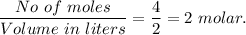
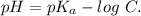 ...1
...1



