
Chemistry, 18.12.2019 19:31 jilliynr9507
If solid nacl is added to a saturated water solution of pbcl2 at 20o c, a precipitate is formed. how would this affect the value of the ksp for [pb2+][cl-] in solution? the ksp increases. the ksp decreases. the ksp remains the same. none of the above.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Note the ph and poh values labeled with letters on the ph scale below. based on log rules and the way ph is calculated, what is the difference in [oh– ] concentration between point a and point b. a) 10^1 b) 10^5 c) 10^6 d) 10^7
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
If solid nacl is added to a saturated water solution of pbcl2 at 20o c, a precipitate is formed. how...
Questions






Mathematics, 21.09.2019 16:30

Mathematics, 21.09.2019 16:30

Mathematics, 21.09.2019 16:30



Computers and Technology, 21.09.2019 16:30




Geography, 21.09.2019 16:30


Geography, 21.09.2019 16:30

Business, 21.09.2019 16:30

Geography, 21.09.2019 16:30

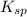 remains the same
remains the same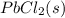 ⇄
⇄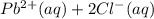
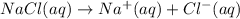
![K_{sp}=[Pb^{2+}][Cl^-]^2](/tpl/images/0424/5289/7fd11.png)
![S=[Pb^{2+}]=\frac{K_{sp}}{[Cl^-]^2}](/tpl/images/0424/5289/c2c31.png)


