

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
If solid nacl is added to a saturated water solution of pbcl2 at 20o c, a precipitate is formed. how...
Questions



Social Studies, 05.08.2019 01:50

Health, 05.08.2019 01:50





History, 05.08.2019 01:50






Mathematics, 05.08.2019 01:50



English, 05.08.2019 01:50


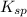 remains the same
remains the same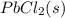 ⇄
⇄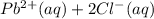
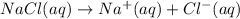
![K_{sp}=[Pb^{2+}][Cl^-]^2](/tpl/images/0424/5297/7fd11.png)
![S=[Pb^{2+}]=\frac{K_{sp}}{[Cl^-]^2}](/tpl/images/0424/5297/c2c31.png)


