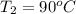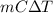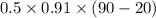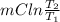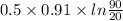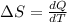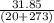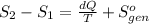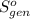Ablock of aluminum with m = 0.5 kg, t = 20oc is dropped into a reservoir at a temperature of 90oc. calculate (a) the change in stored energy (δe), (b) the amount of heat transfer (q), (c) the change in entropy (δs), (d) the amount of entropy transfer by heat and (e) the entropy generation (sgen, univ) in the system's universe during the heat transfer process.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

You know the right answer?
Ablock of aluminum with m = 0.5 kg, t = 20oc is dropped into a reservoir at a temperature of 90oc. c...
Questions

Mathematics, 17.12.2020 21:00





Mathematics, 17.12.2020 21:00


Mathematics, 17.12.2020 21:00



English, 17.12.2020 21:00



Chemistry, 17.12.2020 21:00

Mathematics, 17.12.2020 21:00

Mathematics, 17.12.2020 21:00

Chemistry, 17.12.2020 21:00


Mathematics, 17.12.2020 21:00

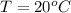 ,
,