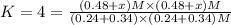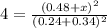Chemistry, 18.12.2019 05:31 djmelodiedaniels
When the reaction co2(g) + h2(g) ⇄ h2o(g) + co(g) is at equilibrium at 1800◦c, the equilibrium concentrations are found to be [co2] = 0.24 m, [h2] = 0.24 m, [h2o] = 0.48 m, and [co] = 0.48 m. then an additional 0.34 moles per liter of co2 and h2 are added. when the reaction comes to equilibrium again at the same temperature, what will be the molar concentration of co?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Your answer should have the same number or significant figures as a he starting measurement. 3201 ml =
Answers: 2

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
You know the right answer?
When the reaction co2(g) + h2(g) ⇄ h2o(g) + co(g) is at equilibrium at 1800◦c, the equilibrium conce...
Questions

Mathematics, 02.07.2021 23:10



Biology, 02.07.2021 23:10


Mathematics, 02.07.2021 23:10



History, 02.07.2021 23:10











History, 02.07.2021 23:10

![[CO_2] = 0.24 M, [H_2] = 0.24 M, [H_2O] = 0.48 M, [CO] = 0.48 M](/tpl/images/0423/7365/45c13.png)
![K=\frac{[H_2O][CO]}{[CO_2][H_2]}=\frac{0.48 M\times 0.48 M}{0.24 M\times 0.24 M}](/tpl/images/0423/7365/2a444.png)
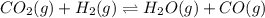
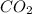 and
and 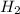 are added.
are added.