Chemistry, 18.12.2019 04:31 angelvega2003
The great french chemist antoine lavoisier discovered the law of conservation of mass in part by doing a famous experiment in 1775. in this experiment lavoisier found that mercury(ii) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas.
1. write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(ii) oxide (hgo) into liquid mercury and gaseous dioxygen.
2. suppose 50.0ml of dioxygen gas are produced by this reaction, at a temperature of 90°c and pressure of exactly 1atm. calculate the mass of mercury(ii) oxide that must have reacted. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
The great french chemist antoine lavoisier discovered the law of conservation of mass in part by doi...
Questions

Biology, 28.07.2019 10:30


Biology, 28.07.2019 10:30



Biology, 28.07.2019 10:30


Biology, 28.07.2019 10:30

English, 28.07.2019 10:30


Mathematics, 28.07.2019 10:30









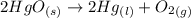
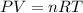
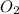 = 0.001677 mol
= 0.001677 mol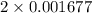 moles of mercury(II) oxide are reacted
moles of mercury(II) oxide are reacted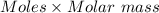 =
=