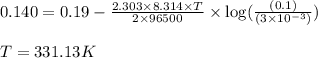An electrochemical cell is constructed such that on one side a pure nickel electrode is in contact with a solution containing ni2+ ions at a concentration of 3 × 10−3 m. the other cell half consists of a pure fe electrode that is immersed in a solution of fe2+ ions having a concentration of 0.1 m. at what temperature will the potential between the two electrodes be +0.140 v?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
You know the right answer?
An electrochemical cell is constructed such that on one side a pure nickel electrode is in contact w...
Questions

Social Studies, 24.11.2019 16:31


Geography, 24.11.2019 16:31

History, 24.11.2019 16:31

History, 24.11.2019 16:31

English, 24.11.2019 16:31

History, 24.11.2019 16:31


Mathematics, 24.11.2019 16:31

History, 24.11.2019 16:31

Social Studies, 24.11.2019 16:31


Biology, 24.11.2019 16:31


Chemistry, 24.11.2019 16:31



Mathematics, 24.11.2019 16:31

English, 24.11.2019 16:31

Mathematics, 24.11.2019 16:31

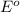 potential will always get reduced and will undergo reduction reaction.
potential will always get reduced and will undergo reduction reaction. 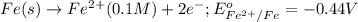
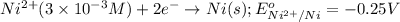
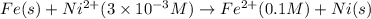
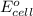 of the reaction, we use the equation:
of the reaction, we use the equation: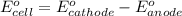
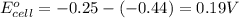
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Fe^{2+}]}{[Ni^{2+}]}](/tpl/images/0423/5757/27fa9.png)
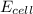 = electrode potential of the cell = +0.140 V
= electrode potential of the cell = +0.140 V![[Fe^{2+}]=0.1M](/tpl/images/0423/5757/e5f8e.png)
![[Ni^{2+}]=3\times 10^{-3}M](/tpl/images/0423/5757/de767.png)