Chemistry, 18.12.2019 02:31 gwendallinesikes
Calculate the change in internal energy (δe) for a system that is absorbing 35.8 kj of heat and is expanding from 8.00 to 24.0 l in volume at 1.00 atm. (remember that 101.3 j = 1 l·atm)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1


Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Calculate the change in internal energy (δe) for a system that is absorbing 35.8 kj of heat and is e...
Questions


Mathematics, 06.04.2021 02:20

English, 06.04.2021 02:20

Computers and Technology, 06.04.2021 02:20


Business, 06.04.2021 02:20


Business, 06.04.2021 02:20




Mathematics, 06.04.2021 02:30







History, 06.04.2021 02:30

Mathematics, 06.04.2021 02:30

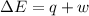
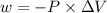
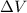 is the change in volume
is the change in volume
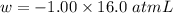
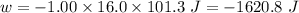 (work is done by the system)
(work is done by the system)