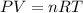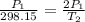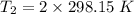Gsuppose a sample of an ideal gas in a container is subjected to a temperature change. a decrease in temperature will the kinetic energy and average speed of the gas particles. as a result, the pressure on the walls of the container will if the gas starts at 25 ∘ c, what temperature would the gas need to reach for its pressure to double? temperature = ∘ c

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1
You know the right answer?
Gsuppose a sample of an ideal gas in a container is subjected to a temperature change. a decrease in...
Questions

Mathematics, 26.03.2020 02:56

Health, 26.03.2020 02:56


Mathematics, 26.03.2020 02:56


Mathematics, 26.03.2020 02:56

Mathematics, 26.03.2020 02:56

Social Studies, 26.03.2020 02:56


Mathematics, 26.03.2020 02:56


Mathematics, 26.03.2020 02:56




Mathematics, 26.03.2020 02:56



Biology, 26.03.2020 02:56

Mathematics, 26.03.2020 02:56