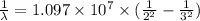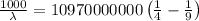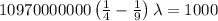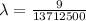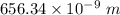The energy of the electron in a hydrogen atom can be calculated from the bohr formula:
e= -r...

Chemistry, 18.12.2019 01:31 tylermdons
The energy of the electron in a hydrogen atom can be calculated from the bohr formula:
e= -ry/n^2
in this equation, ry stands for the rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron.
a) calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=2 to an orbital with n=3 . round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
Questions

Mathematics, 21.09.2019 20:30




Mathematics, 21.09.2019 20:30

Mathematics, 21.09.2019 20:30


History, 21.09.2019 20:30


Computers and Technology, 21.09.2019 20:30

Mathematics, 21.09.2019 20:30



Computers and Technology, 21.09.2019 20:30






Computers and Technology, 21.09.2019 20:30

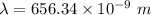
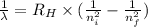
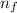 = 3
= 3
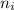 = 2
= 2