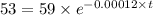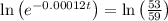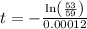The half life for the decay of carbon-14 is 5.73 x 10^3 years. suppose the activity due to the radioactive decay of the carbon-14 in a tiny sample of an artifact made of wood from an archeological dig is measured to be 53.bq. the activity in a similar-sized sample of fresh wood is measured to be 59.bq.
1. calculate the age of the artifact. round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
The half life for the decay of carbon-14 is 5.73 x 10^3 years. suppose the activity due to the radio...
Questions

Mathematics, 08.07.2019 01:20






History, 08.07.2019 01:20

Physics, 08.07.2019 01:20

Mathematics, 08.07.2019 01:20



Biology, 08.07.2019 01:20



Chemistry, 08.07.2019 01:20


Biology, 08.07.2019 01:20

Mathematics, 08.07.2019 01:20

Mathematics, 08.07.2019 01:20

Advanced Placement (AP), 08.07.2019 01:20

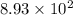 years
years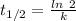
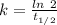
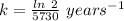
![[A_t]](/tpl/images/0423/2686/5262c.png) = 53 Bq
= 53 Bq![[A_t]=[A_0]e^{-kt}](/tpl/images/0423/2686/1ef89.png)