Using vsepr theory, which of these molecules does not have a trigonal planar shape?
a) ammoni...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
Questions

Mathematics, 04.04.2021 14:00


Chemistry, 04.04.2021 14:00

Chemistry, 04.04.2021 14:00

Mathematics, 04.04.2021 14:00


History, 04.04.2021 14:00


Mathematics, 04.04.2021 14:00



Mathematics, 04.04.2021 14:00

Mathematics, 04.04.2021 14:00


Mathematics, 04.04.2021 14:00

Mathematics, 04.04.2021 14:00

Mathematics, 04.04.2021 14:00

Mathematics, 04.04.2021 14:00

Mathematics, 04.04.2021 14:00

Mathematics, 04.04.2021 14:00

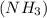 has 3 sigma bonds and one lone pair.Hence its shape is pyramidal due to bond pair - lone pair repulsion.Formaldehyde (HCHO) has 3 sigma bonds and 1 pi bond.Hence it has trigonal planar shape.Sulfur trioxide
has 3 sigma bonds and one lone pair.Hence its shape is pyramidal due to bond pair - lone pair repulsion.Formaldehyde (HCHO) has 3 sigma bonds and 1 pi bond.Hence it has trigonal planar shape.Sulfur trioxide 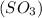 has 3 sigma bonds and 3 pi bonds.Boron triflouride (BF_3) has 3 sigma bonds.
has 3 sigma bonds and 3 pi bonds.Boron triflouride (BF_3) has 3 sigma bonds.

