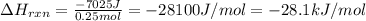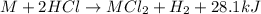Chemistry, 17.12.2019 21:31 colestout2993
Consider the reaction: m + 2hcl → mcl2 + h2
when 0.25 mol of the metal, m, reacted with an aqueous hcl solution (the hcl is in excess), the temperature of the solution rose because the reaction produced 7025 j of heat. what is ∆h in kj per mol of m for this reaction? (hint: is this reaction exothermic or endothermic? )

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 23.06.2019 12:30
Is the genie in the bottle experiment a physical or chemical change/reaction?
Answers: 1
You know the right answer?
Consider the reaction: m + 2hcl → mcl2 + h2
when 0.25 mol of the metal, m, reacted wit...
when 0.25 mol of the metal, m, reacted wit...
Questions

Mathematics, 16.11.2020 21:10

Mathematics, 16.11.2020 21:10

Mathematics, 16.11.2020 21:20

Mathematics, 16.11.2020 21:20


History, 16.11.2020 21:20


Chemistry, 16.11.2020 21:20


English, 16.11.2020 21:20


Physics, 16.11.2020 21:20

Mathematics, 16.11.2020 21:20


History, 16.11.2020 21:20

Mathematics, 16.11.2020 21:20




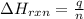
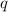 = amount of heat released = -7025 J
= amount of heat released = -7025 J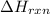 = enthalpy change of the reaction
= enthalpy change of the reaction