
Chemistry, 17.12.2019 17:31 rosemary909
At 297 k , to what pressure can the carbon dioxide in the cartridge inflate a 3.79 l mountain bike tire? (note that the gauge pressure is the difference between the total pressure and atmospheric pressure. in this case, assume that atmospheric pressure is 14.7 psi.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
At 297 k , to what pressure can the carbon dioxide in the cartridge inflate a 3.79 l mountain bike t...
Questions



Mathematics, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20


Chemistry, 18.03.2021 03:20





English, 18.03.2021 03:20



Mathematics, 18.03.2021 03:20


History, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

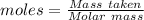
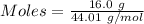
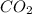 = 0.3636 moles
= 0.3636 moles psi = 37.926 psi
psi = 37.926 psi


