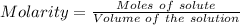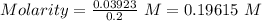Chemistry, 17.12.2019 04:31 simplydimps22owbohb
Asolution of nacl (aq) is added slowly to a solution of lead nitrate, pb(no3)2 ( aq ), until no further precipitation occurs. the precipitate is collected by filtration, dried, and weighed. a total of 10.91 g pbcl2 (s) is obtained from 200.0 ml of the original solution. calculate the molarity of the pb(no3)2 (aq) solution.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
Asolution of nacl (aq) is added slowly to a solution of lead nitrate, pb(no3)2 ( aq ), until no furt...
Questions



History, 12.07.2019 11:30




World Languages, 12.07.2019 11:30



Physics, 12.07.2019 11:30

Mathematics, 12.07.2019 11:30




English, 12.07.2019 11:30

Mathematics, 12.07.2019 11:30




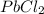 as:-
as:-
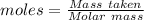
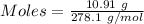
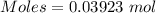
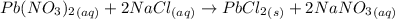
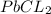 is produced when 1 mole of
is produced when 1 mole of 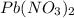 undergoes reaction.
undergoes reaction.