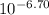Chemistry, 17.12.2019 04:31 Trishamw04
Astudent was given a 50.0 ml of 0.10 m solution of an unknown diprotic acid, h2a, which was titrated with 0.10 m naoh. after a total of 25.0 ml of naoh was added, the resulting solution has a ph of 6.70.
after a total of 50.0 ml of naoh was added, the ph of the solution was 8.00.
determine the values of ka1 and ka2 for the diprotic acid.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2


Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

You know the right answer?
Astudent was given a 50.0 ml of 0.10 m solution of an unknown diprotic acid, h2a, which was titrated...
Questions


Biology, 25.08.2020 07:01


Mathematics, 25.08.2020 07:01

Mathematics, 25.08.2020 07:01

Arts, 25.08.2020 07:01

History, 25.08.2020 07:01


Mathematics, 25.08.2020 07:01

Biology, 25.08.2020 07:01

Mathematics, 25.08.2020 07:01



History, 25.08.2020 07:01

Social Studies, 25.08.2020 07:01



Business, 25.08.2020 07:01

Health, 25.08.2020 07:01