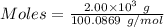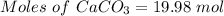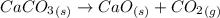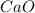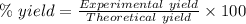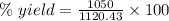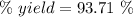Chemistry, 17.12.2019 04:31 lmoleary7466
Quicklime, cao, can be prepared by roasting limestone, caco3, according to the following reaction. caco3(s) ∆→ cao(s) + co2(g). when 2.00 × 103 g caco3 is heated, the actual yield of cao is 1.05 × 103 g. what is the percentage yield?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
Quicklime, cao, can be prepared by roasting limestone, caco3, according to the following reaction. c...
Questions


Mathematics, 06.09.2019 18:30





Biology, 06.09.2019 18:30

Mathematics, 06.09.2019 18:30

Mathematics, 06.09.2019 18:30

English, 06.09.2019 18:30

Physics, 06.09.2019 18:30

Mathematics, 06.09.2019 18:30








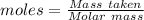
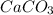 :-
:-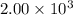 g
g