

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 23.06.2019 10:00
State the effect on the concentration of the clo- ion when there is a decrease in the concentration of the oh- ion
Answers: 1
You know the right answer?
Express the equilibrium constant for the following reaction.2 k(s) + 2 h2o(l) ↔ 2 koh(aq) + h2(g)k =...
Questions


Mathematics, 16.02.2022 14:00












Mathematics, 16.02.2022 14:00

SAT, 16.02.2022 14:00

Spanish, 16.02.2022 14:00

Chemistry, 16.02.2022 14:00



![K=[KOH]^2[H_2]](/tpl/images/0421/6907/b9417.png)
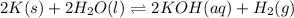
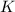 will be,
will be,

