
Chemistry, 17.12.2019 02:31 titofigueroa777
Express the equilibrium constant for the following reaction. p4o10(s) ↔ p4(s) + 5 o2(g)k = [p4][o2]^5/[p4o10]k = [o2]^5k = [p4o10]/[p4][o2]^1/5k = [o2]^-5k = [p4o10]/[p4][o2]^5

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1


You know the right answer?
Express the equilibrium constant for the following reaction. p4o10(s) ↔ p4(s) + 5 o2(g)k = [p4][o2]^...
Questions

Mathematics, 28.08.2020 23:01




Mathematics, 28.08.2020 23:01

Social Studies, 28.08.2020 23:01



English, 28.08.2020 23:01




History, 28.08.2020 23:01

Computers and Technology, 28.08.2020 23:01



Mathematics, 28.08.2020 23:01

Social Studies, 28.08.2020 23:01

Mathematics, 28.08.2020 23:01

History, 28.08.2020 23:01

![K=[O_2]^5](/tpl/images/0421/7050/87eee.png)
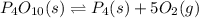
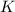 will be,
will be,

