

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

You know the right answer?
For each of the following pairs of substances, determine which has the larger molar entropy at 298 k...
Questions

English, 09.03.2020 14:34

English, 09.03.2020 14:35

English, 09.03.2020 14:36





Mathematics, 09.03.2020 14:37

Physics, 09.03.2020 14:38



Mathematics, 09.03.2020 14:40






Mathematics, 09.03.2020 14:42

Social Studies, 09.03.2020 14:44

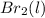 or
or 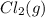 . Since, molar entropy of liquids is less than the molar entropy of gases.
. Since, molar entropy of liquids is less than the molar entropy of gases. 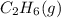 or
or 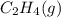 . As both the given species are gaseous in nature. So, more is the molar mass of specie more will be its molar entropy.
. As both the given species are gaseous in nature. So, more is the molar mass of specie more will be its molar entropy.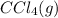 or
or 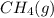 . Molar mass of
. Molar mass of 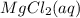 . Molar mass of NaCl 58.44 g/mol and molar mass of
. Molar mass of NaCl 58.44 g/mol and molar mass of 

