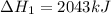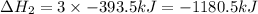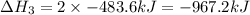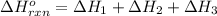Chemistry, 17.12.2019 01:31 gchippewa81
Find the ∆hrxn for the reaction: 3c(s)+4h2(g) →c3h8(g) using these reactions with known ∆h’s: c3h8(g) + 5o2(g) → 3co2(g) + 4h2o(g) ∆h = −2043 kj c(s) + o2(g) → co2(g) ∆h = −393.5 kj 2h2(g) + o2(g) → 2h2o(g) ∆h = −483.6 kj express your answer in kj.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
Find the ∆hrxn for the reaction: 3c(s)+4h2(g) →c3h8(g) using these reactions with known ∆h’s: c3h8...
Questions


Computers and Technology, 05.10.2019 11:30

History, 05.10.2019 11:30

Geography, 05.10.2019 11:30

Mathematics, 05.10.2019 11:30

Mathematics, 05.10.2019 11:30




English, 05.10.2019 11:30

History, 05.10.2019 11:30

Mathematics, 05.10.2019 11:30


English, 05.10.2019 11:30


Mathematics, 05.10.2019 11:30

English, 05.10.2019 11:30


History, 05.10.2019 11:30

Mathematics, 05.10.2019 11:30

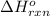 for the reaction is -104.7 kJ.
for the reaction is -104.7 kJ.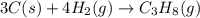 ;
; 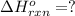
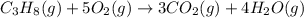 ;
; 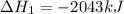
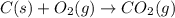 ;
; 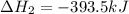
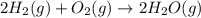 ;
; 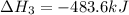
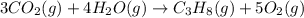 ;
;