

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
Based on the following information, cl2(g) + 2 e- → 2 cl-(aq) e° = + 1.36 v mg2+(aq) + 2 e- → 2 mg(s...
Questions


Social Studies, 12.03.2021 05:40

Mathematics, 12.03.2021 05:40




English, 12.03.2021 05:40




Mathematics, 12.03.2021 05:40

Mathematics, 12.03.2021 05:40



Mathematics, 12.03.2021 05:40

Social Studies, 12.03.2021 05:40


Mathematics, 12.03.2021 05:40


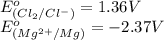
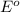 potential will always get reduced and will undergo reduction reaction. Here, chlorine will undergo reduction reaction will get reduced. Magnesium will undergo oxidation reaction and will get oxidized.
potential will always get reduced and will undergo reduction reaction. Here, chlorine will undergo reduction reaction will get reduced. Magnesium will undergo oxidation reaction and will get oxidized.

