
Consider the following reaction at equilibrium. what effect will increasing the temperature have on the system? fe3o4(s) + co(g) ↔ 3 feo(s) + co2(g) δh°= +35.9 kjthe equilibrium constant will decrease. no effect will be observed. the reaction will shift to the right in the direction of products. the equilibrium constant will increase. the reaction will shift to the left in the direction of reactants

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1
You know the right answer?
Consider the following reaction at equilibrium. what effect will increasing the temperature have on...
Questions

Mathematics, 07.07.2019 07:40

History, 07.07.2019 07:40


Biology, 07.07.2019 07:40

Mathematics, 07.07.2019 07:40

English, 07.07.2019 07:40


English, 07.07.2019 07:40


History, 07.07.2019 07:40


Mathematics, 07.07.2019 07:40

Social Studies, 07.07.2019 07:40



History, 07.07.2019 07:40

History, 07.07.2019 07:40


Biology, 07.07.2019 07:40

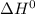 of the given reaction is positive and thus, on increasing temperature, reaction will go in forward direction.
of the given reaction is positive and thus, on increasing temperature, reaction will go in forward direction.

