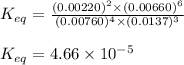9. the first step in industrial nitric acid production is the catalyzed oxidation of ammonia. without a catalyst, a different reaction predominates: 4nh3(g) + 3o2(g) ⇔ 2n2(g) + 6h2o(g) when 0.0120 mol gaseous nh3 and 0.0170 mol gaseous o2 are placed in a 1.00 l container at a certain temperature, the n2 concentration at equilibrium is 2.20×10-3 m. calculate keq for the reaction at this temperature.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1


Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
9. the first step in industrial nitric acid production is the catalyzed oxidation of ammonia. withou...
Questions

Mathematics, 18.12.2020 20:30


Mathematics, 18.12.2020 20:30

Mathematics, 18.12.2020 20:30


Biology, 18.12.2020 20:30



Mathematics, 18.12.2020 20:30

English, 18.12.2020 20:30

Physics, 18.12.2020 20:30

Geography, 18.12.2020 20:30

Mathematics, 18.12.2020 20:30

Biology, 18.12.2020 20:30


Mathematics, 18.12.2020 20:30

Mathematics, 18.12.2020 20:30

Mathematics, 18.12.2020 20:30

Mathematics, 18.12.2020 20:30

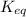 is
is 
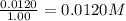
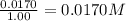
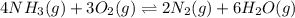
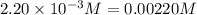
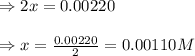
![0.0120-4x=[0.0120-(4\times 0.00110)]=0.00760M](/tpl/images/0421/4577/5e570.png)
![0.0170-3x=[0.0170-(3\times 0.00110)]=0.0137M](/tpl/images/0421/4577/b5ac6.png)
![6x=[6\times 0.00110]=0.00660M](/tpl/images/0421/4577/d0282.png)
![K_{eq}=\frac{[N_2]^2\times [H_2O]^6}{[NH_3]^4\times [O_2]^3}](/tpl/images/0421/4577/7f887.png)