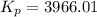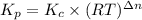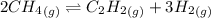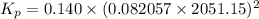Chemistry, 17.12.2019 00:31 jordantay208
For chemical reactions involving ideal gases, the equilibrium constant k can be expressed either in terms of the concentrations of the gases (in m) or as a function of the partial pressures of the gases (in atmospheres). in the latter case, the equilibrium constant is denoted as kp to distinguish it from the concentration-based equilibrium constant kc (sometimes referenced as just k).for the reaction 2ch4(g)⇌c2h2(g)+3h2(g) kc = 0.140 at 1778 ∘c . what is kp for the reaction at this temperature? express your answer numerically.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2



Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
For chemical reactions involving ideal gases, the equilibrium constant k can be expressed either in...
Questions




Mathematics, 12.02.2021 19:00

Mathematics, 12.02.2021 19:00

History, 12.02.2021 19:00






Spanish, 12.02.2021 19:00


Mathematics, 12.02.2021 19:00






Mathematics, 12.02.2021 19:00