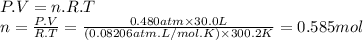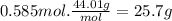Chemistry, 16.12.2019 22:31 cadenhuggins2
Carbon dioxide gas is collected at in an evacuated flask with a measured volume of . when all the gas has been collected, the pressure in the flask is measured to be . calculate the mass and number of moles of carbon dioxide gas that were collected. be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
Carbon dioxide gas is collected at in an evacuated flask with a measured volume of . when all the ga...
Questions


Mathematics, 17.12.2020 22:10

History, 17.12.2020 22:10



Mathematics, 17.12.2020 22:10



English, 17.12.2020 22:10


Mathematics, 17.12.2020 22:10


Engineering, 17.12.2020 22:10



Mathematics, 17.12.2020 22:10

Mathematics, 17.12.2020 22:10

Health, 17.12.2020 22:10


Mathematics, 17.12.2020 22:10